Terpene Profile
Analytes of Interest


Terpenes, the volatile organic compounds responsible for the aroma and flavor profiles of many plants, including cannabis, are remarkably uniform across the botanical kingdom. These compounds are not exclusive to cannabis; they are found in an array of everyday items, from the lemon you might squeeze into your water to the lavender in your soap, and even the pine trees lining your street.
This universality stems from the terpenes’ roles in plant biology — they can attract pollinators with their inviting scents or repel harmful predators with more pungent odors.
Take limonene, for example, with its unmistakable citrus scent prominent in lemons, oranges, and certain cannabis strains, it exemplifies how terpenes bridge our everyday experiences with the plant world. Myrcene, another terpene, offers a musky, earthy aroma familiar to those who enjoy mangoes or the hoppy bitterness of beer, and it's also present in many cannabis varieties, contributing to their distinctive smells and potentially their effects on the body. Pinene, with its refreshing pine scent, is another terpene that links the scent of pine forests to certain cannabis strains, highlighting the interconnectedness of terpene profiles across different species.
Beyond their aromatic qualities, terpenes in various plants, including cannabis, have been studied for their therapeutic properties. For instance, linalool, commonly found in lavender as well as in cannabis, is renowned for its stress-relieving effects. Similarly, eucalyptol, which gives eucalyptus its crisp, cooling scent, appears in the essential oils of some cannabis strains and is valued for its potential anti-inflammatory and analgesic properties.
These aromatic molecules play a critical role in not just the survival and reproductive success of the plants they emanate from but also offer a plethora of uses for humans, ranging from flavoring agents in foods and fragrances in cosmetics to potential therapeutic applications being explored in medical research. The presence of terpenes in cannabis and their shared occurrence in common fruits, herbs, and trees underscore a fascinating biochemical consistency in nature, providing a sensory and functional bridge between our everyday world and the diverse realm of plants.

Myrcene
Major Terpene
Myrcene is characterized by a linear arrangement of ten carbon atoms forming a chain, including a double bond indicative of its unsaturation. This structure is embellished with methyl groups that enhance its volatile, musky aroma.
%20structure.png)
Analyte of Interest
Picture myrcene as a flexible chain of carbon atoms, a bit like a string of pearls, where one pearl is slightly different, representing a double bond that makes myrcene more reactive. This configuration is why myrcene smells earthy and musky, like the ground after rain. It's small and simple, allowing it to slip easily through cell membranes, possibly enhancing the effects of cannabinoids.
Limonene
Major Terpene
Limonene features a cyclic molecule with a six-membered ring and two double bonds, providing its characteristic citrus aroma. The presence of methyl groups attached to the ring structure contributes to its distinct scent.

Analyte of Interest
Limonene is shaped like a ring, similar to a doughnut, with a couple of extra bits sticking out (methyl groups) that make it smell like citrus fruits. This ring structure makes limonene quite stable yet very reactive with air, which is why you can quickly smell an orange once it's peeled. It's nature's way of spreading a refreshing scent, attracting pollinators and repelling predators.
Caryophyllene
Major Terpene
Caryophyllene boasts a unique bicyclic structure with a nine-membered ring fused to a three-membered ring. This configuration includes a rare cyclobutane ring, contributing to its spicy, peppery scent and its ability to interact with cannabinoid receptors.
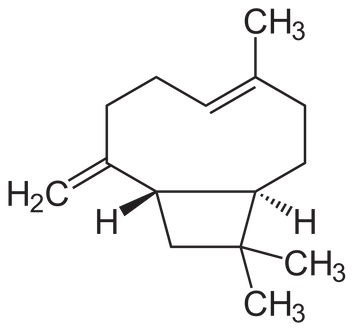
Analyte of Interest
Imagine caryophyllene as two rings stuck together, one larger and one smaller, making it look like a tiny dumbbell. This unique shape lets caryophyllene fit into certain receptors in our body, similar to how a key fits into a lock, explaining its potential to reduce inflammation. Its complex structure gives off a spicy scent, reminiscent of black pepper.
Pinene
Major Terpene
Pinene has a bicyclic structure comprising a six-membered ring and a four-membered ring. This configuration is responsible for its fresh, pine scent and is found in two isomeric forms: alpha-pinene and beta-pinene, differing only in the position of the double bond.

Analyte of Interest
Pinene resembles two connected rings, forming a structure that looks a bit like a twisted ladder. This shape is what makes pinene smell like pine trees, providing that fresh, forest-like aroma. Its structure allows it to interact with various receptors and enzymes in our body, which may help with memory and alertness.
Linalool
Major Terpene
Linalool features a non-cyclic, linear chain with an alcohol functional group, contributing to its floral lavender aroma. Its structure includes two double bonds and a tertiary alcohol group.

Analyte of Interest
Linalool is structured like a straight chain with a small loop at one end, kind of like a lasso. This loop is responsible for its sweet, floral scent, similar to lavender. Linalool's structure makes it particularly good at calming the mind and body, likely by affecting certain brain chemicals.
Ocimene
Minor Terpene
Ocimene is characterized by a monocyclic structure with a eight-membered ring and three double bonds, giving it a sweet, herbal scent. Its structure promotes its volatility and contributes to the terpene's uplifting effects.

Analyte of Interest
Ocimene looks like a zigzag line with a few branches, making it appear as if it's in constant motion. This structure contributes to its sweet, herbaceous aroma, and its shape allows it to act as a powerful decongestant, possibly opening up airways and repelling pests.
Geraniol
Minor Terpene
Geraniol consists of a linear chain with two alcohol groups, endowing it with a sweet, floral aroma reminiscent of roses. The presence of a double bond adds to its reactivity and fragrance profile.

Analyte of Interest
Geraniol is a straight chain with a special end cap (an alcohol group), resembling a wand. This molecular "wand" waves a floral, rose-like fragrance into the air. Its structure helps it play a role in attracting pollinators and potentially offers antioxidant properties.
Eucalyptol
Minor Terpene
Eucalyptol features a cyclic ether with a ten-membered ring, known for its refreshing, cool aroma similar to eucalyptus. Its structure includes an ether bridge, contributing to its minty, cooling sensation.

Analyte of Interest
Eucalyptol forms a ring with an oxygen bridge, looking like a looped belt. This loop gives it a minty, cool scent, much like eucalyptus leaves. Its circular structure helps it dissolve in fats and water, making it a good candidate for medicinal uses, especially for breathing issues.
Bisabolol
Minor Terpene
Bisabolol has a monocyclic structure with a six-membered ring and a methyl group attached to it, along with two alcohol functional groups, which give it a mild, sweet floral aroma.

Analyte of Interest
Bisabolol is a bit twisty, with a couple of rings and a side arm, somewhat resembling a key. Its structure is the reason for its sweet, floral aroma and its ability to soothe and calm, making it a common ingredient in cosmetics for sensitive skin.
Camphene
Minor Terpene
Camphene is formed by two fused cyclic structures, a ten-membered ring system with two double bonds, giving it a firm structure that contributes to its damp, woodsy scent.
-camphene_edited.png)
Analyte of Interest
Camphene's structure looks like two triangles touching at a point, giving it a sharp, woodsy scent. This geometric shape helps camphene act as an antioxidant, potentially protecting cells from damage.
Valencene
Minor Terpene
Valencene has a sesquiterpene structure, characterized by a fifteen-carbon backbone forming a cyclic molecule with three double bonds, offering citrusy, sweet aromas and flavors.

Analyte of Interest
Valencene has a more complex structure, with multiple rings and branches, looking like a tree in full bloom. This complexity is behind its sweet, citrusy aroma, and it's thought to contribute to its ability to repel pests and possibly support skin protection.
